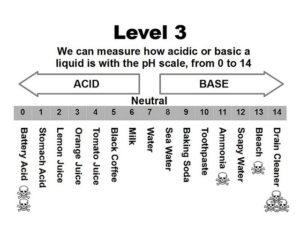
Question: Is vinegar acidic?
Answer: Vinegar is diluted acetic acid. It consists of approximately 5 percent acetic acid by volume. The pH of vinegar is about 2.2 percent, which is close to lemon juice at around a 2 pH. You probably remember from chemistry class that a pH below 7.0 means acidity, 7.0 is neutral. and above 7.0 is a base. Vinegar is considered an acid because its pH is below 7.0.